Asian stock markets are higher today as Chinese and Hong Kong shares show gains. The Shanghai Composite is up 0.05% while the
Hang Seng is up 0.14%. The
Nikkei 225 is trading up by 0.07%. US stocks closed lower in light volume trade on Tuesday as shares of Apple tumbled, offsetting gains in energy stocks as oil prices hit their highest in more than two years.
Pharma stocks opened the day on a mixed note with
Indoco Remedies and
Aarti Drugs being the
most active stocksin this space. As per an article in a leading financial daily, Dr. Reddy's Laboratories Ltd has launched Melphalan Hydrochloride for Injection with the approval of US Food and Drug Administration (USFDA).
The injection is a therapeutic equivalent generic version of Alkeran (melphalan hydrochloride) used in the treatment of certain types of cancer. Alkeran is a registered trademark of Apotex Inc.
According to IMS Health sales data, the Alkeran brand and generic drug had US sales of approximately US$107 million for the most recent twelve months ending in October 2017.
Dr. Reddy's Melphalan Hydrochloride for Injection is available in a carton containing one single-dose clear glass vial of freeze-dried melphalan hydrochloride equivalent to 50 mg melphalan and one 10 mL clear glass vial of sterile diluent.
In another development, Sun Pharmaceutical Industries said the USFDA has accepted a new drug application (NDA), filed by its wholly owned subsidiary, for OTX-101 (cyclosporine A, ophthalmic solution) 0.09%. It is a novel nanomicellar formulation of cyclosporine A 0.09% in a clear, preservative-free aqueous solution.
OTX-101 is now under review for approval by the USFDA, marking an important developmental milestone for Sun Pharma's dry eye candidate.
One shall note that, as per the Indian Pharmaceutical Alliance, the pace of drug approvals has gained momentum after they complained to FDA about delays last year.
Approvals for drugs have also picked up after USFDA concerns at some of the manufacturing units were addressed. Companies such as
Divi's Laboratories,
Cadila Healthcare, Sun Pharmaceuticals and Dr Reddy's Laboratories got regulatory clearances for some of their plants during the year. But several companies are still grappling to resolve FDA concerns.
However, FDA's move to speed up generic drug approvals in a bid to reduce healthcare costs is likely to provide some earnings relief to domestic pharma companies.
After being adversely affected by import bans and the suspension of new drug approvals from manufacturing facilities in the past three years, there has been a sharp
pick-up in new drug approvals in FY17.
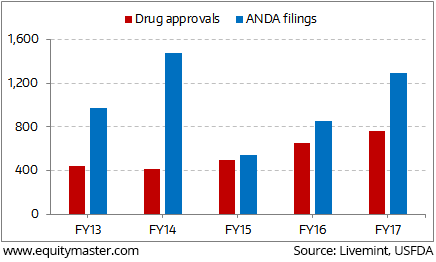
With an aim to lower the overall healthcare costs in the country, USFDA approved a record 763 generic drugs for the financial year ending 30th September. As per Mint Analysis,
Indian pharma companies received 295 approvals accounting for 40% of the overall approvals during the year.
Even the total filings of abbreviated new drug applications (ANDAs) for generic drugs rose to 1,292 in FY17 from 852 in the previous year.
In such an environment, it makes sense for investors to be selective while buying stocks. Focus on value and the underlying fundamentals of the business. Then, they need not worry about the market.
So, what is key to identifying potential multibagger stocks? How does one pick them at the right time and ride them to their full potential? How many multibaggers do you really need to achieve the big riches that you desire?
Most importantly, are there any stocks right now that could turn out to be multibaggers?
Click here to know everything that you need to know right now about mutlibagger stocks...
The decision in this respect was taken at the bank's board meeting held on Tuesday.
The board of bank decided to raise additional equity share capital amounting up to Rs 900 million through QIP route, by issuing up to 90 million equity shares of a face value of Rs 10 each with a premium to be decided as per the applicable guidelines/regulations for an aggregate amount not exceeding Rs 35 billion inclusive of such premium.
A buoyant secondary market, flush with domestic liquidity and the recently announced bank recapitalization plan announced by the central government have resulted in a rush of PSU bank QIPs. Already this month,
Bank of Maharashtra and
Union Bank of India have launched their QIPs.
This year,
QIP fund-raising activity has been dominated by large issuances in the banking and financial services sector. Till November, 33 companies have raised Rs 457.6 billion.
Speaking of QIP route of equity raising, QIPs tend to be a faster way to raise capital as the dealing happens with a few investors - only institutions in this case. And this is why companies prefer this route - because of its convenience and fewer resource requirements compared to other methods of raising equity.

No comments:
Post a Comment