Asian share markets are positive today as Japanese and Hong Kong shares rise. The Nikkei 225 is up 0.6% while the Hang Seng is up 0.3%. The Shanghai Composite is trading down by 0.1%.
Back home, India share markets opened the day on a positive note. The BSE Sensex is higher by 105 points while the NSE Nifty is trading up by 30 points. The BSE Mid Cap index and BSE Small Cap index both opened the day up by 0.6% & 0.9% respectively.
Sectoral indices have opened the day on a positive note with pharma stocks and stocks in the consumer durables sector witnessing maximum buying interest. The rupee is trading at 67.35 to the US$.
In news from the realty sector. New accounting rules could burn a hole in the pockets of real estate developers. According to a leading financial daily, the implementation of a new accounting standard from this fiscal will force listed real estate companies to write back profits made over the past few years from all incomplete projects.
Under the Indian Accounting Standard 115 (IND-AS 115), applicable from this financial year, real estate companies will have to write back over Rs 200 billion from their net worth.
Real estate companies will have to switch to the Project Completion Method from the existing Percentage Completion Method (POC).
Under the previous standards, buyer payments toward the purchase of under construction flats were declared as revenues by companies and net income generated from such projects was treated as profit.
This could affect the balance sheets of realty companies, many of whom still suffer under the burden of high debt.
Reportedly, some developers have written to the government seeking relief.
The BSE Realty Index opened the day up 0.5%.
Moving on to news from stocks in the pharma sector. Sun Pharma share price is in focus today after the US Food and Drug Administration (USFDA) updated the status of its Halol facility to voluntary action initiated (VAI) on Friday.
VAI, which means that though objectionable conditions were found at the plant, the issues do not justify further regulatory action and any corrective action is left to the USFDA to take voluntarily.
This may come as a positive development for the company as the Halol Plant contributes the maximum to the company’s US revenues.
Expediting Drug Approval Process to be a Positive for Industry
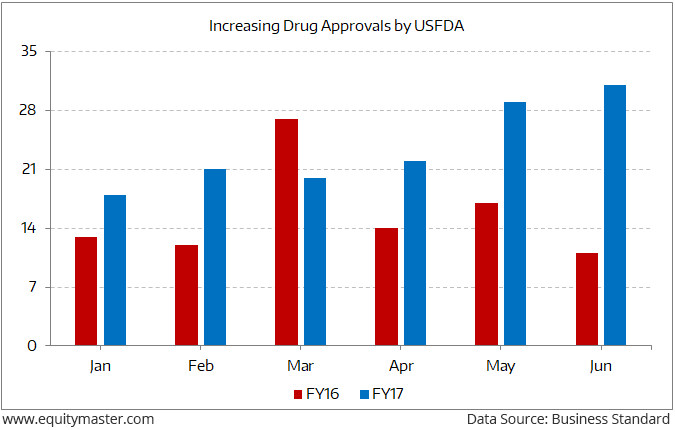
Indian pharma companies catering to the US markets are breathing a sigh of relief. After being adversely affected by import bans and the suspension of new drug approvals from manufacturing facilities in the past three years, there has been a sharp pick-up in new drug approvals in FY17.
However, note that USFDA alerts on Indian pharma companies have increased over the past few years. Regulators used to visit the plants every two years. Now they come every eight months. Increasing inspections have led to a total of 41 import alerts in the past eight years — 33 of them (80%) in just the last four years (2013–16). This clearly signifies increased USFDA scrutiny on Indian pharma firms. If that wasn’t enough, increasing pricing pressure in the generics segment has dented realisations.
However, the recent development of USFDA expediting the drug approval process can bring some respite for Indian pharma companies. This comes as drug approvals for Indian companies have gone up 50% in the period from January to June 2017 compared to the same period last year.
While short-term pain is expected, companies with strong R&D capabilities and compliant plants will do well over the long term. The uncertainties make it important to be stock specific in the sector. It is important to look for companies that have the competence and staying power to overcome the challenges.
This article was Originally published at www.equitymaster.com.
No comments:
Post a Comment